FDA approves first treatment to regrow hair in teens with alopecia areata
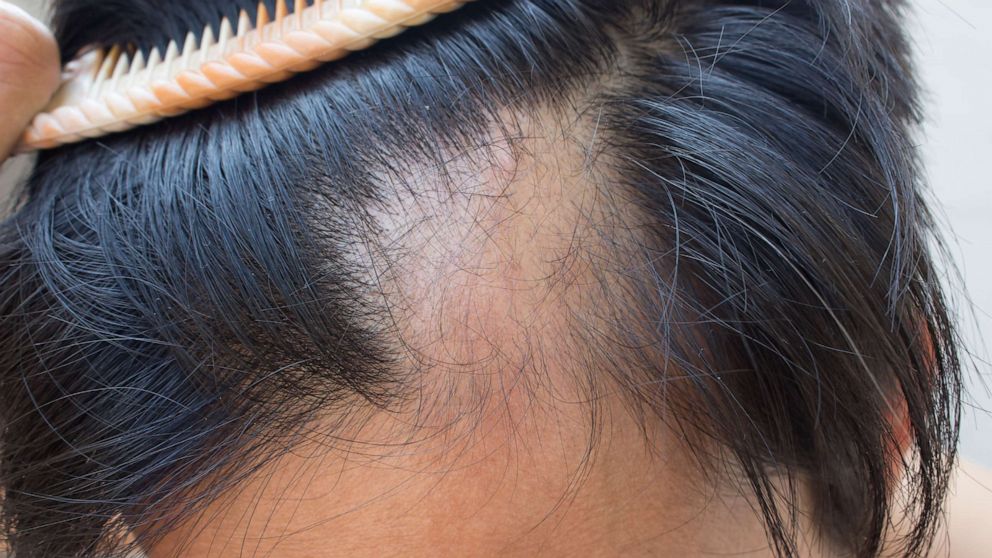
The US Food and Drug Administration (FDA) approved a hair loss treatment for children last Friday, marking a historic first.
Ritlecitinib is a once-daily pill for children 12 years of age and older with severe alopecia areata, a condition in which the body attacks hair follicles and causes hair loss.
The drug is marketed under the brand name Litfulo and is manufactured by Pfizer.
Pfizer said Litfulo will be available to consumers “in the next few weeks.”
According to Pfizer, a one-year supply of Litfulo has a list price of $49,000, just like other specialty dermatological treatments. The company said the actual cost for patients will depend on their individual health care plan.
“We remain committed to helping patients access the treatments they need,” Pfizer said in a statement. “There will be co-pay savings for commercially insured patients and a patient assistance program for eligible patients to help achieve this. Through the Pfizer Dermatology Patient Access Program, eligible patients will have access to LITFULO for assistance. can.”
The drug was already a game changer for Maria Strattner, an 18-year-old with alopecia who took part in a clinical trial that led to FDA approval.
Maria Stratner from Danbury, Connecticut lost all her hair, including her eyelashes and eyebrows, in two weeks at the age of 13 and was diagnosed with severe alopecia areata.
Her mother, Maryann Strattner, told ABC News that her daughter was struggling emotionally and physically with her hair falling out. She said they were determined to find a treatment that worked together.
Maryann Strattner said, “As a parent, all you want for your kids is to be happy and healthy. Period.” She said, “So if you see a hairless kid coming to you at 13, she’ll know how to get help.”
Maryann Strattner said her daughter discovered clinical trials for ritlecitinib at Yale University in New Haven, Connecticut, and entered trials in 2020.
According to Maria Stratner’s mother, her hair started growing back within a few months of taking the medication. Her mother said of her daughter’s persistence, “She’s lucky she’s smart enough not to give up.”
According to Maryann Strattner, Maria Stratner previously had blonde hair, but her hair grew brown back into taut waves.
Dr. Brett King, associate professor of dermatology at Yale University School of Medicine and lead researcher on the Littlecitinib trial, said this might happen in some cases with drugs that make people’s hair grow differently.
He described the FDA’s approval of ritlecitinib as a “tremendous advance” in the treatment of alopecia, and described the drug’s effectiveness as “nearly transformative”.
“During the 24 weeks of treatment, about 30% of the people in the trial regrew their hair,” King told ABC. “Remember, these are patients who initially had 50% to 100% scalp hair loss,” King said. No,” he said. News. “And after 24 weeks, 30% of them were less than 20%. [scalp hair loss] or complete scalp hair regrowth, and that number increases to 40% of those who achieve dramatic hair regrowth by up to 48 weeks.”
MORE: Will Smith vs Chris Rock face off at the Oscars spotlights alopecia, a condition that affects millions.
Dr. King emphasized that littlelecitinib is considered a treatment for alopecia, not a cure. He said patients are expected to continue taking the drug in the long term to maintain hair growth.
Littlecitinib is a class of drugs known as JAK inhibitors, a new type of drug that “interferes with the body’s signals thought to cause inflammation,” according to the American Academy of Dermatology Association.
King noted that patients with a history of cancer, blood clots or cardiovascular disease should “consider very carefully” the use of littlecitinib.
“Like all medicines in its class, a class of medicines called JAK inhibitors, Ritlecitinib has a caveat,” King said. “Of course, these warnings should be taken into account by everyone. And when we think about taking medications… through a careful decision-making thought process and consideration with patients, we can ensure that this treatment is absolutely appropriate and safe for patients. Very well identifiable.”
In addition to being approved for use in children 12 years of age and older, ritlecitinib is FDA-approved for use in adults with alopecia areata.
Last year, the FDA also approved Olumiant, a once-daily pill for adults with alopecia areata.
What you need to know about alopecia
There are several types of alopecia, the collective term for hair loss.
Experts don’t fully understand the biochemical processes of all these conditions, but according to the National Institutes of Health, some types occur when a person’s immune system inappropriately targets their own hair follicles to suppress hair growth, while others: I think it can be like It is caused by genetics, hormones or certain diseases such as hypothyroidism or hyperthyroidism.
Experts believe that a combination of environmental and genetic factors can cause disease.
Alopecia areata is a condition that occurs when the body specifically attacks hair follicles, causing hair loss. According to the AADA, among the subtypes are generalized alopecia, totalis alopecia, and partial alopecia areata.
According to the NIH, generalized alopecia, the complete loss of all body, facial, and scalp hair, is considered the most extreme and rarest form of the condition.
Alopecia totalis, characterized by loss of hair only on the scalp, is a less advanced form of the condition.
Spotted alopecia areata causes small, circular patches of baldness, usually on the scalp and face.
MORE: Meet the little girl who inspires her to wear a pink wig every year in her small town
This is the most common type of alopecia areata, according to the NIH.
Alopecia areata affects nearly 2 percent of the general population, or nearly 7 million people in the United States, at some point in their lifetime, according to the National Alopecia Areata Foundation, a California-based nonprofit organization.
According to the NIH, the condition affects men and women similarly and affects all racial and ethnic groups.
Most people with alopecia areata are affected in their teens, 20s, or 30s, but it can occur at any age, says the NIH.
#FDA #approves #treatment #regrow #hair #teens #alopecia #areata





